The science behind munchies: cannabis and your appetite
Tetrahydrocannabinol (THC), a cannabinoid in cannabis, can bind the cannabinoid receptor type 1 (CB1) and thus promote overeating and weight gain. This effect, detrimental to most people, can prove beneficial under certain medical conditions, such as cancer and HIV-associated wasting syndrome.
Last Updated:January 31, 2023
Cannabis (aka marijuana) is a popular recreational drug that also has a variety of potential medicinal uses, from boosting appetite to reducing nausea and anxiety.[1] In cancer patients, cannabis may help ease the pain associated with chemotherapy while simultaneously increasing appetite (a serious side effect of chemotherapy being nausea).[1]
Aside from euphoria, this increase in appetite is probably cannabis best-known effect — you might refer to it as “the munchies”. Historical sources indicate that people as early as 300 BCE knew that cannabis stimulated appetite, especially for sweet and savory food.[2]

But what exactly is cannabis and how does it affect appetite? Let’s dig in.
How cannabis works
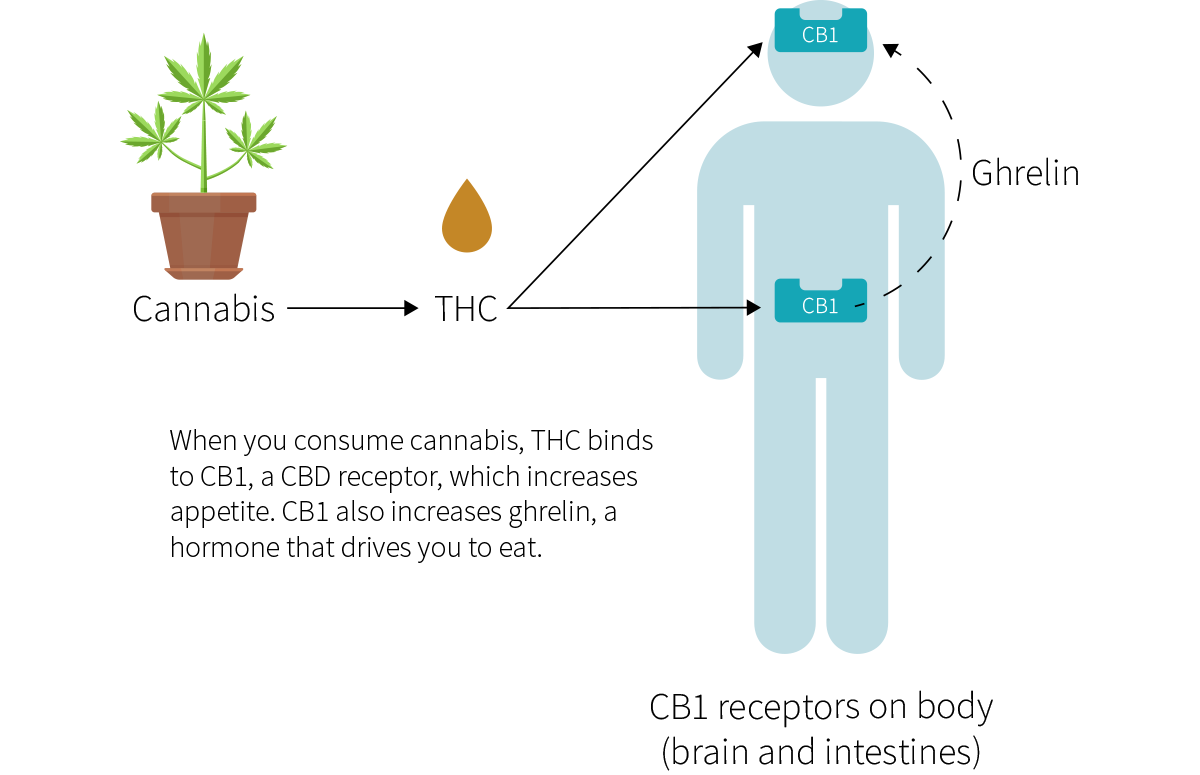
The word “cannabis” refers to several plants in the Cannabis genus, including sativa, indica, and ruderalis. The cannabinoid compound tetrahydrocannabinol (THC), one of cannabis main active ingredients, is also the main reason why cannabis can cause “the munchies”: by partially binding to and thus activating cannabinoid receptor type 1 (CB1), THC increases your appetite.
In the different body tissues it occupies, CB1 acts in slightly different ways, several of which increase appetite.[2] CB1 can be found in the following areas:
- The basal ganglia, where it may enhance eating pleasure.
- The limbic forebrain, where it may enhance food palatability.
- The stomach and small intestine, which both regulate ghrelin (an appetite-stimulating hormone that speeds digestion).
- The hypothalamus and rhombencephalon, two sections of the brain that help regulate food intake.
By activating CB1, THC increases appetite through the following known mechanisms:
- It may decrease your levels of peptide tyrosine tyrosine (PYY), thus increasing your levels of ghrelin, thus increasing your appetite.[3]
- It activates the mTOR (mammalian target of rapamycin) pathway, thus increasing your levels of ghrelin, thus increasing your appetite.[4][5]
- It activates a subset of neurons called proopiomelanocortin neurons (POMCs). These neurons can suppress hunger (primary pathway) and/or increase appetite (secondary pathway), to various degrees. Recent research on CB1 has revealed that dronabinol, a synthetic form of THC, can stimulate the secondary pathway without stimulating the primary one.[6]
The method of THC consumption (inhalational, oral, sublingual, or rectal) can affect caloric intake and might influence food choice (preference for sweet, salty, sour, or bitter foods).[7] For example, suppositories led to greater daily caloric intake than did oral capsules. The differences in appetite stimulation are caused mostly by differences in potency and rate of absorption — two factors modulated by the consumption method, the co-consumption of other substances, and the tolerance of the subject.[1]
THC, one of cannabis main active ingredients, can interact with receptors that can, in turn, increase appetite. The method of consumption can affect how THC influences food choice and overall food intake, as can the amount and nature of the products consumed concurrently.
Does cannabis cause weight gain?
Few studies have looked at cannabis effect on the weight of healthy subjects, and the results are conflicting.
Cross-sectional observational studies are lower-quality studies that collect data at a single point in time. One such study associated cannabis with greater fat mass,[8] but the others associated it with a smaller waist,[9] lower BMI,[10][11] and lower prevalence of obesity.[12] Even a higher-quality prospective observational study found no association between extensive cannabis use and higher BMI.[13]
Keep in mind, however, that those studies all relied on self-reported use, and that such reports are always, to some extent, unreliable. Studies that used more reliable methods paint a different picture. Three clinical trials (whose participants were largely confined to a controlled hospital environment) saw increases in body weight.[14][15][16] However, all three trials were relatively short-term and had modest sample sizes.
Cannabis was associated with little or no weight gain in observational studies, but with weight gain in a few higher-quality studies (clinical trials).
Cannabis for weight gain in clinical populations
While many people wish they could lose weight, people with HIV-associated wasting syndrome, cancer-associated cachexia, or anorexia nervosa, notably, are often underweight. Two synthetic THC-based drugs have been developed to address the issue: nabilone (Cesamet®) and dronabinol (Marinol®, Syndros®).
HIV-associated wasting syndrome
- The unintentional, steady weight loss associated with HIV can lead to poor health outcomes.
- Oral cannabinoids (dronabinol) may help increase weight but the results are not entirely conclusive.[17][18]
- The various doses used by the different studies may have been lower than optimal.[19]
Cancer-associated cachexia
- The loss of skeletal muscle associated with cancer is called cancer-associated cachexia, or simply cancer wasting.[20]
- In two large RCTs, 2.5 mg of dronabinol (synthetic THC) twice daily increased body weight, but not enough to improve health.[21][22]
- This 5 mg daily dose, like the doses used in the HIV-wasting studies, may have been too small to elicit a beneficial effect.
Anorexia nervosa
- Two small, short RCTs have investigated THC as a treatment option for anorexia nervosa.[23][24]
- People in the intervention groups received 2.5 mg of dronabinol twice daily for 4 weeks.
- People in the intervention groups gained, on average, 0.70 kg[24] and 0.73 kg[23] (1.54 lb and 1.6 lb) more than people in the placebo groups. This increase is small, but it may still benefit this population.
THC, natural or synthetic, may increase body weight in people with HIV-associated wasting syndrome. In people with anorexia nervosa or cancer-associated cachexia, it might have the same effect, but the evidence is weaker.
CB1: the key to weight loss?
Since activating CB1 increases appetite, it isn’t surprising that blocking this receptor has the opposite effect. Studies on individual cells show that blocking CB1 increases the production of adiponectin,[25] an anti-inflammatory hormone negatively associated with obesity.[26]
Researchers have used endocannabinoid antagonists (compounds that block CB1) to treat obesity caused by compulsive binging or irresistible cravings for sweets and snacks. Rats given the anti-obesity drug rimonabant, an endocannabinoid antagonist, lost weight and experienced a reduction in their blood levels of insulin.[27] Human trials of the same drug showed weight losses of 2.6–6.3 kg (5.7–13.7 lb) above placebo.[28] Most of these trials lasted over a year, including one that saw a plateau at 9 months.[29]
Yet in spite of these successes, rimonabant failed to earn approval from the U.S. Food and Drug Administration (FDA) and was withdrawn from the European market,[30] due to side effects that include nausea, dizziness, severe depression, and suicidal thoughts.[31][32]
Since CB1s are found throughout the body, it is difficult to pinpoint the causes and mechanisms of these side effects. Still, in the future, safer endocannabinoid antagonists may play a role in treating obesity by blocking CB1 in order to increase adiponectin production and reduce appetite.
Drugs that block CB1, such as rimonabant, lead to significant weight loss, but the side effects are severe. Still, future studies on endocannabinoid antagonists (i.e., CB1 blockers) might lead to breakthroughs in the fight against obesity.
Bottom line
By activating CB1, a receptor present in various body tissues, the cannabinoid THC in cannabis can increase appetite.
Current evidence suggests that regular cannabis use promotes weight gain.
THC might be useful for increasing body weight in some clinical populations (such as people with HIV wasting, cancer wasting, or anorexia nervosa).
Drugs that block CB1 promote weight loss but can also cause many harmful side effects.
Should you wish to learn more about cannabis, please visit our dedicated supplement page.
