Vitamin K
Vitamin K is an essential vitamin found in plants or produced from intestinal bacteria. It plays an essential role in bone health and regulates blood clotting.
Vitamin K is most often used for
Last Updated:April 1, 2024
Vitamin K is an essential vitamin. It is one of the four fat-soluble vitamins, along with vitamin A, vitamin D, and vitamin E. It was named vitamin K after the German word koagulation, because vitamin K’s role in blood coagulation was first discovered in Germany. Vitamin K can be found in dark green vegetables, matcha tea, and natto (a type of fermented soybeans). Vitamin K can also be found in various animal products.
The Recommended Daily Intake (RDI) of vitamin K is sufficient to support healthy blood coagulation. Higher levels of vitamin K, however, may provide additional benefits. Unfortunately, it can be difficult to obtain high levels of vitamin K from food alone. Most people don’t like natto enough to eat 50g a day, so supplementation of vitamin K is a popular option.
Vitamin K has at times been found to improve markers of bone health. Despite this, supplementation has inconsistent effects on bone fracture risk.
Vitamin K has been suggested to protect cardiovascular health by reducing the calcification and stiffening of arteries. However, a number of clinical trials have found no apparent effect of vitamin K supplementation on arterial calcification. Most of these trials were on people with extensive arterial calcification already present, meaning additional research is needed to determine if vitamin K can prevent tissue calcification before it has started.
Vitamin K may have a role to play in cancer therapy. It may also help with regulating insulin sensitivity and (in topical form) may help reduce skin reddening, but more research is needed before strong conclusions can be drawn.
Vitamin K’s main mechanism is through the vitamin K cycle, which is a cyclical metabolic pathway that uses vitamin K to modify various proteins. Specifically, vitamin K is used to attach a carbon dioxide molecule onto a glutamate contained in these proteins, allowing the protein to bind calcium ions. These proteins can then utilize calcium ions for various biological processes.
Vitamin K is often supplemented alongside vitamin D, since vitamin D also supports bone health. In fact, taking both together may improve the effects of each, since they are believed to work synergistically. Excessive vitamin D can lead to arterial calcification and in animal models vitamin K reduces this buildup.
- Phylloquinone
- Menaquinone
- MK-4
- MK-7
- Menatetrenone
- Phytonadione
- Pyrroloquinoline quinone (sounds similar to phylloquinone
- totally different molecule)
Vitamin K comes in a variety of different forms, known as vitamers. Forms of vitamin K are either phylloquinones (vitamin K1) or menaquinones (vitamin K2). There are different vitamers within the vitamin K2 class, abbreviated as MK-x.
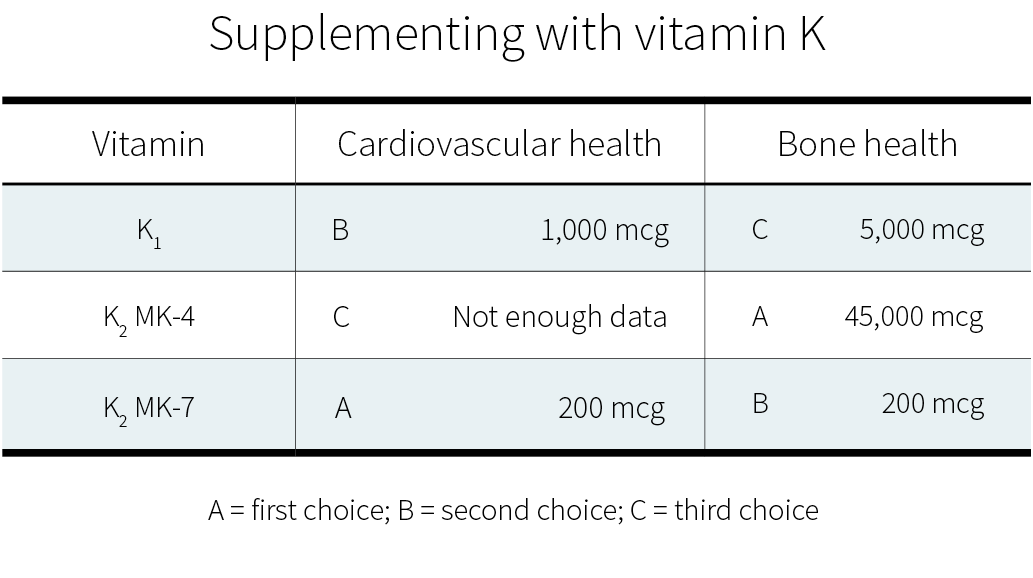
The minimum effective dose for phylloquinone (vitamin K1) is 50 μg, which is enough to satisfy the Recommended Daily Intake (RDI) for vitamin K. The maximum dose for vitamin K1 is 10,000 μg (10 mg).
The minimum effective dose for short chain menaquinones (MK-4) is 1,500 μg. Doses of up to 45 mg (45,000 μg) have been safely used in a superloading dosing protocol.
The minimum effective dose for longer chain menaquinones (MK-7, MK-8, and MK-9) is between 90 and 360 μg. Further research is needed to determine the maximum effective dose for MK-7.
A topical application of vitamin K should contain at least 5% phylloquinone.
Vitamin K should be supplemented alongside fatty acids, even if the vitamin is coming from a plant-based source, so consider taking vitamin K at meal time. Microwaving plant-based sources of vitamin K will increase the absorption rate of the vitamin.
Research is still scarce, but current evidence suggests that, through their effect on calcium regulation, some forms of vitamin K can help prevent osteoporosis and cardiovascular diseases.
Vitamin K is poorly understood, both by the general public and among health professionals. It has a wide range of potential benefits, but their nature and extent are still uncertain.
Why is that?
Some vitamins are more popular than others. In the past, a lot of research went into vitamin C, which became a popular supplement. Nowadays, a lot of research goes into vitamin D, whose popularity as a supplement is steadily growing.
By contrast, research on vitamin K is still scarce, having slowly developed over the past two decades. Further, it is scattered, because there exist several forms of vitamin K. Some of those forms are present only in a few foods. Others exist in various foods, but only in minute amounts. Few have been the subject of human trials.
The human trials that do exist, however, are overall promising. In order to understand their value and limitations, first you need to know a few basic facts. So let’s begin:
What is vitamin K?
Of the four fat-soluble vitamins (A, D, E, and K), vitamin K was discovered last. In 1929, Danish scientist Henrik Dam discovered a compound that played a role in coagulation (blood clotting).[1] When he first published his findings, in a German journal, he called this compound Koagulationsvitamin, which became known as vitamin K.
Today, we know that vitamin K participates in some very important biological processes, notably the carboxylation of calcium-binding proteins (including osteocalcin and matrix GLA protein).[2] In other words, vitamin K helps modify proteins so they can bind calcium ions (Ca2+). Through this mechanism, vitamin K partakes in blood clotting, as Henrik Dam discovered, but also of calcium regulation: it helps ensure that more calcium gets deposited in bones and less in soft tissues, thus strengthening bones and reducing arterial stiffness.
What complicates matters is that each vitamin has different forms, called vitamers, each of which may affect you differently. Vitamin K has natural vitamers, K1 (phylloquinone) and K2 (menaquinone), and synthetic vitamers, the best-known of which is K3 (menadione).
Vitamin K1
K1 is produced in plants, where it is involved in photosynthesis: the greener the plant, the greater its chlorophyll content; the greater its chlorophyll content, the greater its K1 content. When it comes to foods, K1 is especially abundant in green leafy vegetables.
K1 makes for 75–90% of the vitamin K in the Western diet.[3] Unfortunately, K1 is tightly bound to chloroplasts (organelles that contain chlorophyll and conduct photosynthesis), so you could be absorbing very little of what you eat[4] — maybe less than 10%.[5] Since vitamin K is a fat-soluble vitamin, however, its absorption can be enhanced by the co-ingestion of fat: adding fat to cooked spinach can raise K1 bioavailability from 5% to 13%.[6]
Vitamin K2
Things become more complicated here, because just as there are several forms of vitamin K, there are several forms of vitamin K2. To be more precise, the side chain of K1 always has four isoprenoid units (five-carbon structures), so there is only one form of K1, but the side chain of K2 has n isoprenoid units, so there are n forms of K2, called MK-n.[7][8]
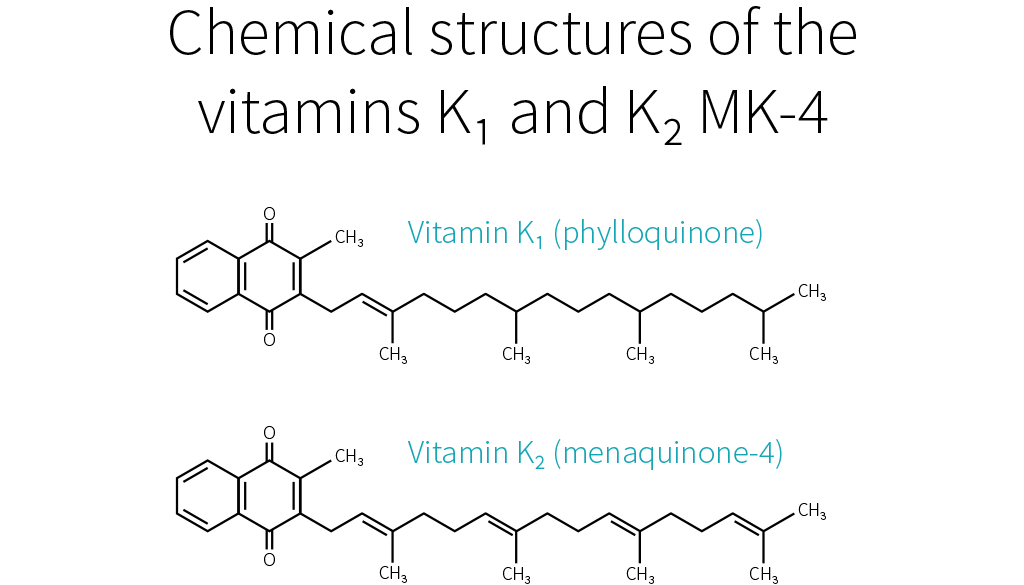
Whereas the side chain of K1 has four saturated isoprenoid units, the side chain of K2 MK-4 has four unsaturated isoprenoid units. Although K1 is directly active in your system, your body can also convert it to MK-4.[9][10][11] How much gets converted depends notably on your genetic heritage.[3]
MK-4 is present in animal products (meat, eggs, and dairy), though only in small quantities. Because those foods usually contain fat, dietary MK-4 should be better absorbed than dietary K1,[12] but future studies will need to confirm this hypothesis.

Other than MK-4, all forms of K2 are produced by bacteria. Your microbiota was once thought to produce three-fourths of the vitamin K you absorb.[13] Vitamin K, however, is mostly produced in the colon, where there are no bile salts to facilitate its absorption, so the actual ratio is probably much lower.[12][14]
Bacteria-produced K2 can be found in fermented foods, such as cheese and curds, but also in liver meat.[15] The richest dietary source of K2 is natto (fermented soybeans), which contains mostly MK-7.[16][17] As it stands, MK-7 is the only form of K2 that can be consumed in supplemental doses through food (i.e., natto). For that reason, MK-7 is the most-studied form of K2, together with MK-4.
K1 and MK-4 both have a side chain composed of four isoprenoid units; their half-life in your blood is 60–90 minutes. MK-7 has a side chain composed of seven isoprenoid units; it remains in your blood for several days. Due to their different side-chain lengths, the various forms tend to be transported on different lipoproteins, which are taken up at different rates by various tissues.[18][19][20][21][22] K1 and MK-4 are used quickly (K1 in the liver, MK-4 in other specific tissues), whereas MK-7 has more time to travel and be used throughout the body (which makes it, in theory, the best option for bone health).
Vitamin K3
K1 and K2 are the only natural forms of vitamin K, but there exist several synthetic forms, the best known of which is K3. However, whereas the natural forms of vitamin K are safe, even in high doses, K3 can interfere with glutathione, your body’s main antioxidant. K3 was once used to treat vitamin K deficiency in infants, but it caused liver toxicity, jaundice, and hemolytic anemia. Nowadays, it is used only in animal feed, in small doses. In the animals, vitamin K3 gets converted into K2 MK-4,[23] which you can consume safely.
Vitamin K is a family of fat-soluble vitamins. K1 and K2, the natural forms, are safe even in high doses. There is only one type of K1; it is found in plants, notably green leafy vegetables; your body can use it directly or convert it to K2 MK-4. Aside from MK-4, all other types of K2 are produced by bacteria, including the bacteria populating your gut. MK-4 is present in animal products (meat, eggs, dairy), whereas other types of K2 can be found in fermented foods and liver meat.
Vitamin K and your health
As far as we know, vitamin K mainly affects bloodclotting, cardiovascular health, and bone health. Epidemiological studies have mostly focused on K1; cardiovascular trials, on K1 and MK-7 (the main type present in natto, the richest dietary source of K2); bone trials, on MK-4 (the type of K2 your body can make out of K1).
Blood clotting
Vitamin K deficiency impairs blood clotting, causing excessive bleeding and bruising. It is rare in adults, but more common in newborns (more than 4 cases per 100,000 births in the UK[24]), where it can result in life-threatening bleeding within the skull. For that reason, the American Academy of Pediatrics recommends that newborns receive K1 shortly after birth (intramuscular injections have shown greater efficacy than oral administration).[25]
If you suffer from hypercoagulation (if your blood clots too easily), you might be prescribed a vitamin K antagonist (VKA), such as warfarin, a medication that hinders the recycling of vitamin K. Some doctors recommend that VKA users shun vitamin K entirely, but preliminary evidence suggests that, under professional supervision, vitamin K supplements might help stabilize the effects of VKAs.[15]
Which form should be supplemented, though, and in what amount, is still uncertain. There is some evidence that K1 enhances coagulation more than does MK-4[26][27] but less than does MK-7.[20] With regard to daily supplementation, 100 μg of K1 is considered safe, but in some people 10 μg of MK-7 is enough to significantly impair VKA therapy.[28]
Remember that natto is rich in MK-7. A single serving of natto can increase blood clotting for up to four days,[29] so it is one food VKA users should avoid. Other foods should be safe to eat. Please note that in people who do not suffer from hypercoagulation, and thus do not need to medicate with VKA, high intakes of natto have never been correlated to excessive blood clotting. Similarly, human studies saw no increase in blood-clot risk even from 45 mg (45,000 μg) of MK-4 taken once[30] or even thrice[31] daily.
Cardiovascular health
As we saw, vitamin K partakes in calcium regulation: it helps ensure that more calcium gets deposited in bones and less in soft tissues, thus reducing arterial stiffness. This is why people who take vitamin K antagonists, such as warfarin, are more likely to suffer from vascular calcification.[32][33]
Epidemiological studies[34][35][36] and mechanistic evidence[37] suggest that dietary K2 benefits cardiovascular health more than an equal dose of dietary K1.
Clinical trials on supplemental vitamin K have focused on K1[38][39] and MK-7.[40][41][42] Often, those trials used a combination of vitamin D and other nutrients, but with vitamin K being the key difference between the intervention group and the control groups. Both of these forms of vitamin K seem to cause a consistent reduction in arterial stiffness (with better evidence for MK-7), and less consistent reductions in coronary calcification and carotid intima-media thickness. Judging from those trials and the epidemiological evidence, MK-7 seems the better choice.
Bone health
As we have just seen again, vitamin K partakes in calcium regulation: it helps ensure that less calcium gets deposited in soft tissues and more in bones, thus strengthening the latter. This is why people who take vitamin K antagonists, such as warfarin, might be more at risk of bone fractures,[43][44] though not all studies agree they are.[45]
Current evidence suggests that supplementing with vitamin K — or, at least, with certain forms of vitamin K — can benefit bone health, especially in the elderly (who have lower levels of circulating K2).[46] This potential should be explored, since, as the world population grows (and grows older), so does the number of osteoporotic fractures.[47] [48][49]
MK-7 appears to support the carboxylation of osteocalcin (a major calcium-binding protein in bones) more efficiently than K1.[20] Clinical trials suggest that, for the purpose of increasing bone density, MK-4 and MK-7 work more reliably than K1.[50]
More significantly, a meta-analysis of MK-4 trials found an overall decrease in fracture risk.[51] The effect of K1 or MK-7 supplementation on fracture risk is less clear. Only one K1 trial looked at fracture risk; it reported a decrease, but without a concomitant increase in bone mineral density.[52] Of the two MK-7 trials, one reported no difference in the number of fractures between the placebo group and the MK-7 group,[53] whereas the other reported fewer fractures in the MK-7 group;[54] there were, however, no statistical analyses for either study.
More research on vitamin K and fracture risk will be needed to clarify the effects of the different forms at different dosages. Currently, if you wish to supplement for bone health, a very high dose of MK-4 (45,000 μg) is the option best supported by human studies.[51] Those studies, all in Japanese people, focused on the prevention of bone fractures, and yes, much smaller dosages can probably help support bone health; but how much smaller?
In a 12-month study, 20 patients suffering from a chronic kidney disorder were given a daily glucocorticoid (a corticosteroid that has for side effect to decrease bone formation and increase bone resorption). In addition, half the patients received 15 mg of MK-4 daily, while the other half received a placebo. The placebo group experienced bone-density loss (BDL) in the lumbar spine, while the MK-4 group did not.[55]
More recently, a 12-month study in 48 postmenopausal Japanese women gave 1.5 mg of MK-4 daily to half of them and found a significant reduction in forearm BDL, but not in hip BDL, and it didn’t evaluate fractures.[56]
So there is some evidence for dosages lower than 45 mg/day. It is, however, a lot weaker.
In healthy people, vitamin K supplementation does not increase the risk of blood clots. Judging from limited evidence, MK-7 seems to be the best form of vitamin K for cardiovascular health, and MK-4 the best form of vitamin K for bone health.
How much vitamin K do you need?
Since vitamin K is crucial to your health, why is it the subject of relatively few studies? One of the reasons is simply that vitamin K deficiency is very rare in healthy, well-fed adults. It is mostly a concern in newborns, in people who have been prescribed a vitamin K antagonist, in people who suffer from severe liver damage, and in people who have problems absorbing fat.[57][58][59]
Vitamin K is abundant in a balanced diet, and the bacteria in your colon can also produce some. Moreover, your body can recycle it many times, and this vitamin K-epoxide cycle more than makes up for the limited ability your body shows for storing vitamin K.
Still, you can recycle vitamin K many times, but not forever, and so you still need to consume some regularly. But how much, exactly?
No one knows. There is, as yet, not enough evidence to set a Recommended Dietary Allowance (RDA) for vitamin K, so an Adequate Intake (AI) has been established at a level assumed to prevent excessive bleeding. In the United States, the AI for vitamin K is 120 μg/day for men and 90 μg/day for women. In Europe, the AI for vitamin K is 70 μg/day for men and women. More recent research, however, suggests that those numbers should be increased.[19]
Since 100 g of collards contain, on average, 360 μg of vitamin K,[60][7] getting enough vitamin K looks easy. But can’t you just as easily get too much?
Fortunately, no. Though allergic reactions have occurred with vitamin K injections,[61][62][63] no incidence of actual toxicity has ever been reported in people taking natural vitamin K, even in high supplemental doses.[64] For that reason, neither the FDA nor the EFSA has set a Tolerable Upper Intake Level (UL) for vitamin K. One should note, however, that we lack long-term, high-dose studies on vitamin K safety.
Sources of vitamin K
K1 can be found in plant products, notably green leafy vegetables. K2 MK-4 can be found in animal products (meat, eggs, and dairy). The other types of K2 can be found in fermented foods and liver meat.

Table references: [65][66][7][67][68][22][60][69][70]
Meats’ vitamin K content correlates positively but non-linearly with their fat content and will vary according to the animal’s diet (and thus country of origin). Forms of K2 other than MK-4 and MK-7 have not been well studied but are likely to have some benefit — cheeses and beef liver are notable sources of others forms of K2[66][70] and cheese consumption is associated with a reduced risk of cardiovascular disease.[71]
While well-conducted controlled trials provide the most reliable evidence, most such trials used amounts of vitamin K2 that far exceed what could be obtained through foods, save for natto. This leaves us wondering if dietary K2 has any effect.
Fortunately, it seems to be the case: a high dietary intake of K2 (≥33 μg/day seems optimal) may reduce the risk of coronary heart disease — an effect a high dietary intake of K1 doesn’t appear to have.[34][36][35][72] It doesn’t mean, of course, that foods rich in K1 are valueless: dietary K1 intake will protect you from excessive bleeding and is inversely associated with risk of bone fractures.[73]
Observational studies, however, are less reliable than controlled trials, so we know less about the effects of dietary intake than about the effects of supplemental intake. If you wish to supplement with vitamin K, here are the dosages supported by the current evidence:
Summary
Although much more research needs to be performed, there is early evidence that vitamin K, whether in food or in supplemental form, can benefit cardiovascular health and bone health.

🚧 Under Renovation 🚧
The information in this section is slated for renovation — it will soon be transformed into a more usable (and readable!) form in the coming months. As such, the text in this section may be out of date and not up to Examine’s current standards for writing style.
- ^Dam HThe antihaemorrhagic vitamin of the chickBiochem J.(1935 Jun)
- ^Booth SLRoles for vitamin K beyond coagulationAnnu Rev Nutr.(2009)
- ^Shearer MJ, Newman PRecent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesisJ Lipid Res.(2014 Mar)
- ^Garber AK, Binkley NC, Krueger DC, Suttie JWComparison of phylloquinone bioavailability from food sources or a supplement in human subjectsJ Nutr.(1999 Jun)
- ^Gijsbers BL, Jie KS, Vermeer CEffect of food composition on vitamin K absorption in human volunteersBr J Nutr.(1996 Aug)
- ^Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti GOral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice GuidelinesChest.(2012 Feb)
- ^Booth SLVitamin K: food composition and dietary intakesFood Nutr Res.(2012)
- ^Kurosu M, Begari EVitamin K2 in electron transport system: are enzymes involved in vitamin K2 biosynthesis promising drug targets?Molecules.(2010 Mar 10)
- ^Shearer MJ, Newman PMetabolism and cell biology of vitamin KThromb Haemost.(2008 Oct)
- ^Davidson RT, Foley AL, Engelke JA, Suttie JWConversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteriaJ Nutr.(1998 Feb)
- ^Ronden JE, Drittij-Reijnders MJ, Vermeer C, Thijssen HHIntestinal flora is not an intermediate in the phylloquinone-menaquinone-4 conversion in the ratBiochim Biophys Acta.(1998 Jan 8)
- ^Beulens JW, Booth SL, van den Heuvel EG, Stoecklin E, Baka A, Vermeer CThe role of menaquinones (vitamin K₂) in human healthBr J Nutr.(2013 Oct)
- ^Miggiano GA, Robilotta LVitamin K-controlled diet: problems and prospectsClin Ter.(2005 Jan-Apr)
- ^Ichihashi T, Takagishi Y, Uchida K, Yamada HColonic absorption of menaquinone-4 and menaquinone-9 in ratsJ Nutr.(1992 Mar)
- ^Holmes MV, Hunt BJ, Shearer MJThe role of dietary vitamin K in the management of oral vitamin K antagonistsBlood Rev.(2012 Jan)
- ^Ikeda Y, Iki M, Morita A, Kajita E, Kagamimori S, Kagawa Y, Yoneshima HIntake of fermented soybeans, natto, is associated with reduced bone loss in postmenopausal women: Japanese Population-Based Osteoporosis (JPOS) StudyJ Nutr.(2006 May)
- ^Katsuyama H, Ideguchi S, Fukunaga M, Saijoh K, Sunami SUsual dietary intake of fermented soybeans (Natto) is associated with bone mineral density in premenopausal womenJ Nutr Sci Vitaminol (Tokyo).(2002 Jun)
- ^Sato T, Schurgers LJ, Uenishi KComparison of menaquinone-4 and menaquinone-7 bioavailability in healthy womenNutr J.(2012 Nov 12)
- ^Vermeer CVitamin K: the effect on health beyond coagulation - an overviewFood Nutr Res.(2012)
- ^Schurgers LJ, Teunissen KJ, Hamulyák K, Knapen MH, Vik H, Vermeer CVitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7Blood.(2007 Apr 15)
- ^Schurgers LJ, Vermeer CDifferential lipoprotein transport pathways of K-vitamins in healthy subjectsBiochim Biophys Acta.(2002 Feb 15)
- ^Schurgers LJ, Vermeer CDetermination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrationsHaemostasis.(2000 Nov-Dec)
- ^Nakagawa K, Hirota Y, Sawada N, Yuge N, Watanabe M, Uchino Y, Okuda N, Shimomura Y, Suhara Y, Okano TIdentification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzymeNature.(2010 Nov 4)
- ^Shearer MJVitamin KLancet.(1995 Jan 28)
- ^American Academy of Pediatrics Committee on Fetus and NewbornControversies concerning vitamin K and the newborn. American Academy of Pediatrics Committee on Fetus and NewbornPediatrics.(2003 Jul)
- ^Spronk HM, Soute BA, Schurgers LJ, Thijssen HH, De Mey JG, Vermeer CTissue-specific utilization of menaquinone-4 results in the prevention of arterial calcification in warfarin-treated ratsJ Vasc Res.(2003 Nov-Dec)
- ^Groenen-van Dooren MM, Soute BA, Jie KS, Thijssen HH, Vermeer CThe relative effects of phylloquinone and menaquinone-4 on the blood coagulation factor synthesis in vitamin K-deficient ratsBiochem Pharmacol.(1993 Aug 3)
- ^Theuwissen E, Teunissen KJ, Spronk HM, Hamulyák K, Ten Cate H, Shearer MJ, Vermeer C, Schurgers LJEffect of low-dose supplements of menaquinone-7 (vitamin K2 ) on the stability of oral anticoagulant treatment: dose-response relationship in healthy volunteersJ Thromb Haemost.(2013 Jun)
- ^Schurgers LJ, Shearer MJ, Hamulyák K, Stöcklin E, Vermeer CEffect of vitamin K intake on the stability of oral anticoagulant treatment: dose-response relationships in healthy subjectsBlood.(2004 Nov 1)
- ^Ushiroyama T, Ikeda A, Ueki MEffect of continuous combined therapy with vitamin K(2) and vitamin D(3) on bone mineral density and coagulofibrinolysis function in postmenopausal womenMaturitas.(2002 Mar 25)
- ^Asakura H, Myou S, Ontachi Y, Mizutani T, Kato M, Saito M, Morishita E, Yamazaki M, Nakao SVitamin K administration to elderly patients with osteoporosis induces no hemostatic activation, even in those with suspected vitamin K deficiencyOsteoporos Int.(2001 Dec)
- ^Mayer O Jr, Seidlerová J, Bruthans J, Filipovský J, Timoracká K, Vaněk J, Cerná L, Wohlfahrt P, Cífková R, Theuwissen E, Vermeer CDesphospho-uncarboxylated matrix Gla-protein is associated with mortality risk in patients with chronic stable vascular diseaseAtherosclerosis.(2014 Jul)
- ^Chatrou ML, Winckers K, Hackeng TM, Reutelingsperger CP, Schurgers LJVascular calcification: the price to pay for anticoagulation therapy with vitamin K-antagonistsBlood Rev.(2012 Jul)
- ^Gast GC, de Roos NM, Sluijs I, Bots ML, Beulens JW, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PH, van der Schouw YTA high menaquinone intake reduces the incidence of coronary heart diseaseNutr Metab Cardiovasc Dis.(2009 Sep)
- ^Beulens JW, Bots ML, Atsma F, Bartelink ML, Prokop M, Geleijnse JM, Witteman JC, Grobbee DE, van der Schouw YTHigh dietary menaquinone intake is associated with reduced coronary calcificationAtherosclerosis.(2009 Apr)
- ^Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JCDietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam StudyJ Nutr.(2004 Nov)
- ^El Asmar MS, Naoum JJ, Arbid EJVitamin k dependent proteins and the role of vitamin k2 in the modulation of vascular calcification: a reviewOman Med J.(2014 May)
- ^Shea MK, O'Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, Price PA, Williamson MK, Booth SLVitamin K supplementation and progression of coronary artery calcium in older men and womenAm J Clin Nutr.(2009 Jun)
- ^Braam LA, Hoeks AP, Brouns F, Hamulyák K, Gerichhausen MJ, Vermeer CBeneficial effects of vitamins D and K on the elastic properties of the vessel wall in postmenopausal women: a follow-up studyThromb Haemost.(2004 Feb)
- ^Kurnatowska I, Grzelak P, Masajtis-Zagajewska A, Kaczmarska M, Stefańczyk L, Vermeer C, Maresz K, Nowicki MEffect of vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3-5Pol Arch Med Wewn.(2015)
- ^Knapen MH, Braam LA, Drummen NE, Bekers O, Hoeks AP, Vermeer CMenaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trialThromb Haemost.(2015 May)
- ^Fulton RL, McMurdo ME, Hill A, Abboud RJ, Arnold GP, Struthers AD, Khan F, Vermeer C, Knapen MH, Drummen NE, Witham MDEffect of Vitamin K on Vascular Health and Physical Function in Older People with Vascular Disease--A Randomised Controlled TrialJ Nutr Health Aging.(2016 Mar)
- ^Caraballo PJ, Heit JA, Atkinson EJ, Silverstein MD, O'Fallon WM, Castro MR, Melton LJ 3rdLong-term use of oral anticoagulants and the risk of fractureArch Intern Med.(1999 Aug 9-23)
- ^Gage BF, Birman-Deych E, Radford MJ, Nilasena DS, Binder EFRisk of osteoporotic fracture in elderly patients taking warfarin: results from the National Registry of Atrial Fibrillation 2Arch Intern Med.(2006 Jan 23)
- ^Jamal SA, Browner WS, Bauer DC, Cummings SRWarfarin use and risk for osteoporosis in elderly women. Study of Osteoporotic Fractures Research GroupAnn Intern Med.(1998 May 15)
- ^Hodges SJ, Pilkington MJ, Shearer MJ, Bitensky L, Chayen JAge-related changes in the circulating levels of congeners of vitamin K2, menaquinone-7 and menaquinone-8Clin Sci (Lond).(1990 Jan)
- ^Pisani P, Renna MD, Conversano F, Casciaro E, Di Paola M, Quarta E, Muratore M, Casciaro SMajor osteoporotic fragility fractures: Risk factor updates and societal impactWorld J Orthop.(2016 Mar 18)
- ^Dhanwal DK, Dennison EM, Harvey NC, Cooper CEpidemiology of hip fracture: Worldwide geographic variationIndian J Orthop.(2011 Jan)
- ^Johnell O, Kanis JAAn estimate of the worldwide prevalence and disability associated with osteoporotic fracturesOsteoporos Int.(2006 Dec)
- ^Fang Y, Hu C, Tao X, Wan Y, Tao FEffect of vitamin K on bone mineral density: a meta-analysis of randomized controlled trialsJ Bone Miner Metab.(2012 Jan)
- ^Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJVitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trialsArch Intern Med.(2006 Jun 26)
- ^Cheung AM, Tile L, Lee Y, Tomlinson G, Hawker G, Scher J, Hu H, Vieth R, Thompson L, Jamal S, Josse RVitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): a randomized controlled trialPLoS Med.(2008 Oct 14)
- ^Emaus N, Gjesdal CG, Almås B, Christensen M, Grimsgaard AS, Berntsen GK, Salomonsen L, Fønnebø VVitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trialOsteoporos Int.(2010 Oct)
- ^Knapen MH, Drummen NE, Smit E, Vermeer C, Theuwissen EThree-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal womenOsteoporos Int.(2013 Sep)
- ^Sasaki N, Kusano E, Takahashi H, Ando Y, Yano K, Tsuda E, Asano YVitamin K2 inhibits glucocorticoid-induced bone loss partly by preventing the reduction of osteoprotegerin (OPG)J Bone Miner Metab.(2005)
- ^Koitaya N, Sekiguchi M, Tousen Y, Nishide Y, Morita A, Yamauchi J, Gando Y, Miyachi M, Aoki M, Komatsu M, Watanabe F, Morishita K, Ishimi YLow-dose vitamin K2 (MK-4) supplementation for 12 months improves bone metabolism and prevents forearm bone loss in postmenopausal Japanese womenJ Bone Miner Metab.(2013 May 24)
- ^Nowak JK, Grzybowska-Chlebowczyk U, Landowski P, Szaflarska-Poplawska A, Klincewicz B, Adamczak D, Banasiewicz T, Plawski A, Walkowiak JPrevalence and correlates of vitamin K deficiency in children with inflammatory bowel diseaseSci Rep.(2014 Apr 24)
- ^Jagannath VA, Fedorowicz Z, Thaker V, Chang ABVitamin K supplementation for cystic fibrosisCochrane Database Syst Rev.(2013 Apr 30)
- ^Nakajima S, Iijima H, Egawa S, Shinzaki S, Kondo J, Inoue T, Hayashi Y, Ying J, Mukai A, Akasaka T, Nishida T, Kanto T, Tsujii M, Hayashi NAssociation of vitamin K deficiency with bone metabolism and clinical disease activity in inflammatory bowel diseaseNutrition.(2011 Oct)
- ^Booth SL, Pennington JA, Sadowski JAFood sources and dietary intakes of vitamin K-1 (phylloquinone) in the American diet: data from the FDA Total Diet StudyJ Am Diet Assoc.(1996 Feb)
- ^Shiratori T, Sato A, Fukuzawa M, Kondo N, Tanno SSevere Dextran-Induced Anaphylactic Shock during Induction of Hypertension-Hypervolemia-Hemodilution Therapy following Subarachnoid HemorrhageCase Rep Crit Care.(2015)
- ^Riegert-Johnson DL, Volcheck GWThe incidence of anaphylaxis following intravenous phytonadione (vitamin K1): a 5-year retrospective reviewAnn Allergy Asthma Immunol.(2002 Oct)
- ^Bullen AW, Miller JP, Cunliffe WJ, Losowsky MSSkin reactions caused by vitamin K in patients with liver diseaseBr J Dermatol.(1978 May)
- ^Institute of Medicine (US) Panel on MicronutrientsDietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc
- ^Fu X, Shen X, Finnan EG, Haytowitz DB, Booth SLMeasurement of Multiple Vitamin K Forms in Processed and Fresh-Cut Pork Products in the U.S. Food SupplyJ Agric Food Chem.(2016 Jun 8)
- ^Manoury E, Jourdon K, Boyaval P, Fourcassié PQuantitative measurement of vitamin K2 (menaquinones) in various fermented dairy products using a reliable high-performance liquid chromatography methodJ Dairy Sci.(2013 Mar)
- ^Kamao M, Suhara Y, Tsugawa N, Uwano M, Yamaguchi N, Uenishi K, Ishida H, Sasaki S, Okano TVitamin K content of foods and dietary vitamin K intake in Japanese young womenJ Nutr Sci Vitaminol (Tokyo).(2007 Dec)
- ^Elder SJ, Haytowitz DB, Howe J, Peterson JW, Booth SLVitamin k contents of meat, dairy, and fast food in the u.s. DietJ Agric Food Chem.(2006 Jan 25)
- ^Shimogawara K, Muto SPurification of Chlamydomonas 28-kDa ubiquitinated protein and its identification as ubiquitinated histone H2BArch Biochem Biophys.(1992 Apr)
- ^Hirauchi K, Sakano T, Notsumoto S, Nagaoka T, Morimoto A, Fujimoto K, Masuda S, Suzuki YMeasurement of K vitamins in animal tissues by high-performance liquid chromatography with fluorimetric detectionJ Chromatogr.(1989 Dec 29)
- ^Chen GC, Wang Y, Tong X, Szeto IMY, Smit G, Li ZN, Qin LQCheese consumption and risk of cardiovascular disease: a meta-analysis of prospective studiesEur J Nutr.(2017 Dec)
- ^Villines TC, Hatzigeorgiou C, Feuerstein IM, O'Malley PG, Taylor AJVitamin K1 intake and coronary calcificationCoron Artery Dis.(2005 May)
- ^Hao G, Zhang B, Gu M, Chen C, Zhang Q, Zhang G, Cao XVitamin K intake and the risk of fractures: A meta-analysisMedicine (Baltimore).(2017 Apr)
- Bone Mineral Density - Iwamoto I, Kosha S, Noguchi S, Murakami M, Fujino T, Douchi T, Nagata YA longitudinal study of the effect of vitamin K2 on bone mineral density in postmenopausal women a comparative study with vitamin D3 and estrogen-progestin therapyMaturitas.(1999 Jan 4)
- Bone Mineral Density - Braam LA, Knapen MH, Geusens P, Brouns F, Vermeer CFactors affecting bone loss in female endurance athletes: a two-year follow-up studyAm J Sports Med.(2003 Nov-Dec)
- Bone Mineral Density - Knapen MH, Schurgers LJ, Vermeer CVitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal womenOsteoporos Int.(2007 Jul)
- Bone Mineral Density - Koitaya N, Sekiguchi M, Tousen Y, Nishide Y, Morita A, Yamauchi J, Gando Y, Miyachi M, Aoki M, Komatsu M, Watanabe F, Morishita K, Ishimi YLow-dose vitamin K2 (MK-4) supplementation for 12 months improves bone metabolism and prevents forearm bone loss in postmenopausal Japanese womenJ Bone Miner Metab.(2013 May 24)
- Bone Mineral Density - Binkley N, Harke J, Krueger D, Engelke J, Vallarta-Ast N, Gemar D, Checovich M, Chappell R, Suttie JVitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American womenJ Bone Miner Res.(2009 Jun)
- Bone Mineral Density - Booth SL, Dallal G, Shea MK, Gundberg C, Peterson JW, Dawson-Hughes BEffect of vitamin K supplementation on bone loss in elderly men and womenJ Clin Endocrinol Metab.(2008 Apr)
- Bone Mineral Density - Knapen MH, Drummen NE, Smit E, Vermeer C, Theuwissen EThree-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal womenOsteoporos Int.(2013 Sep)
- Bone Mineral Density - Shea MK, Dallal GE, Dawson-Hughes B, Ordovas JM, O'Donnell CJ, Gundberg CM, Peterson JW, Booth SLVitamin K, circulating cytokines, and bone mineral density in older men and womenAm J Clin Nutr.(2008 Aug)
- Bone Mineral Density - Ushiroyama T, Ikeda A, Ueki MEffect of continuous combined therapy with vitamin K(2) and vitamin D(3) on bone mineral density and coagulofibrinolysis function in postmenopausal womenMaturitas.(2002 Mar 25)
- Bone Mineral Density - Nishiguchi S, Shimoi S, Kurooka H, Tamori A, Habu D, Takeda T, Kubo SRandomized pilot trial of vitamin K2 for bone loss in patients with primary biliary cirrhosisJ Hepatol.(2001 Oct)
- Liver Cancer Risk - Ishizuka M, Kubota K, Shimoda M, Kita J, Kato M, Park KH, Shiraki TEffect of menatetrenone, a vitamin k2 analog, on recurrence of hepatocellular carcinoma after surgical resection: a prospective randomized controlled trialAnticancer Res.(2012 Dec)
- Liver Cancer Risk - Toshihiko Mizuta, Iwata Ozaki, Yuichiro Eguchi, Tsutomu Yasutake, Seiji Kawazoe, Kazuma Fujimoto, Kyosuke YamamotoThe effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment: a pilot studyCancer.(2006 Feb 15)
- Fracture Risk - Shiraki M, Shiraki Y, Aoki C, Miura MVitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosisJ Bone Miner Res.(2000 Mar)
- Bone Mineral Density - Forli L, Bollerslev J, Simonsen S, Isaksen GA, Kvamsdal KE, Godang K, Gadeholt G, Pripp AH, Bjortuft ODietary vitamin K2 supplement improves bone status after lung and heart transplantationTransplantation.(2010 Feb 27)
- Bone Mineral Density - Purwosunu Y, Muharram, Rachman IA, Reksoprodjo S, Sekizawa AVitamin K2 treatment for postmenopausal osteoporosis in IndonesiaJ Obstet Gynaecol Res.(2006 Apr)
- Bone Mineral Density - Sasaki N, Kusano E, Takahashi H, Ando Y, Yano K, Tsuda E, Asano YVitamin K2 inhibits glucocorticoid-induced bone loss partly by preventing the reduction of osteoprotegerin (OPG)J Bone Miner Metab.(2005)
- Bone Mineral Density - Braam LA, Knapen MH, Geusens P, Brouns F, Hamulyák K, Gerichhausen MJ, Vermeer CVitamin K1 supplementation retards bone loss in postmenopausal women between 50 and 60 years of ageCalcif Tissue Int.(2003 Jul)
- Estrogen - Ozuru R, Sugimoto T, Yamaguchi T, Chihara KTime-dependent effects of vitamin K2 (menatetrenone) on bone metabolism in postmenopausal womenEndocr J.(2002 Jun)
- Glycemic Control - Hyung Jin Choi, MD, Juyoun Yu, BS, Hosanna Choi, BS, Jee Hyun An, MD, Sang Wan Kim, MD, PHD, Kyong Soo Park, MD, PHD, Hak C. Jang, MD, PHD, Seong Yeon Kim, MD, PHD and Chan Soo Shin, MD, PHDVitamin K2 Supplementation Improves Insulin Sensitivity via Osteocalcin Metabolism: A Placebo-Controlled Trial2011.()
- Insulin - Sakamoto N, Nishiike T, Iguchi H, Sakamoto KPossible effects of one week vitamin K (menaquinone-4) tablets intake on glucose tolerance in healthy young male volunteers with different descarboxy prothrombin levelsClin Nutr.(2000 Aug)
- Erythema - W W Lou, A T Quintana, R G Geronemus, M C GrossmanEffects of topical vitamin K and retinol on laser-induced purpura on nonlesional skinDermatol Surg.(1999 Dec)
- Erythema - Cohen JL, Bhatia ACThe role of topical vitamin K oxide gel in the resolution of postprocedural purpuraJ Drugs Dermatol.(2009 Nov)
- Bruising - Shah NS, Lazarus MC, Bugdodel R, Hsia SL, He J, Duncan R, Baumann LThe effects of topical vitamin K on bruising after laser treatmentJ Am Acad Dermatol.(2002 Aug)
- Bruising - Kovács RK, Bodai L, Dobozy A, Kemény LLack of the effect of topical vitamin K on bruising after mechanical injuryJ Am Acad Dermatol.(2004 Jun)










