Betalains
Betalains are a group of reddish pigments found in some fruits where the red anthocyanin compounds (such as pelargonidin) are replaced with betalains; a major component of beetroot, it can dye the urine a reddish tint.
Betalains is most often used for
Last Updated:September 28, 2022
1.
Sources and Structure
1.1
Sources
Betalains are nitrogen containing pigments found in a variety of foods that are reddish in hue, and occur in red plants that do not contain anthocyanin molecules as the red pigment (ie. beet root contains betalains, whereas strawberries contains anthocyanins such as pelargonidin for their reddish hue).[1] They are at times used for food coloration in products such as ice cream, candies, and processed meat[1][2] and the quantity in these foods rarely exceeds 50mg/kg due to their strong pigment coloration[3] stronger even than red radish anthocyanins.[4] Betalains in general as a food colorant, since they almost exclusively come from beets, are usually referred to as 'beetroot red' and are considered a natural food colorant.[2]
The majority of plants containing betalains are in the caryophyllales family with exception to the caryophyllaceae and molluginaceae genera, which accumulate anthocyanins for their red coloration.[5]
Betalains are reddish-purple pigments that are found in some plants when anthocyanins are nor present as pigmentation
Common dietary sources of betalains include:
- Beet root products (Beta vulgaris subspecies vulgaris)[6] with the content in red beets ranging from 0.02-0.21% depending on cultivar[2] with the major red components being betanidin and betanin (as well as their isomers) while the major yellow components are vulgaxanthin I and vulgaxanthin II[7]
- Colored swiss chard (Beta vulgaris subspecies cicla)[8]
- Amaranth (Amaranthus genus)[9]
- Cactus fruits (Opuntia and hylocereus genera)[10][6] and pitahaya (Hylocereus)[11]
1.2
Structure
All the betalains are divided into two groups based on the color they confer, either the betacyanins (purple reddish) or the betaxanthins (yellow orangish), with betanin and indicaxanthin being the major components for each category respectively.[1]
Betalains, as a group, are based off the chromophore (core of the molecule which causes it to be a pigment) known as betalamic acid[1] and two major betalains are indicaxanthin (betalamic acid connected to the amino acid proline) and betanidin where a catechol group is attached to said proline.[1]
Many betalains are actually glycosides (bound to sugar) or acylglycosides (bound to acetylated sugars) of betanidin,[1] of which one of the most common is a simple glucoside (bound to a single glucose molecule) known as betanin;[1] others include phyllocactin (6'-O-Malonylbetanin), 2',O-Apiosylphyllocactin, 2'-(5"-O-E-Feruloylapiosyl)betanin, 2'-(5"-O-E-Feruloylapiosyl)phyllocactin, amaranthin, _gomphrenin_s, _portulacaxanthin_s, and hylocerenin.[1]
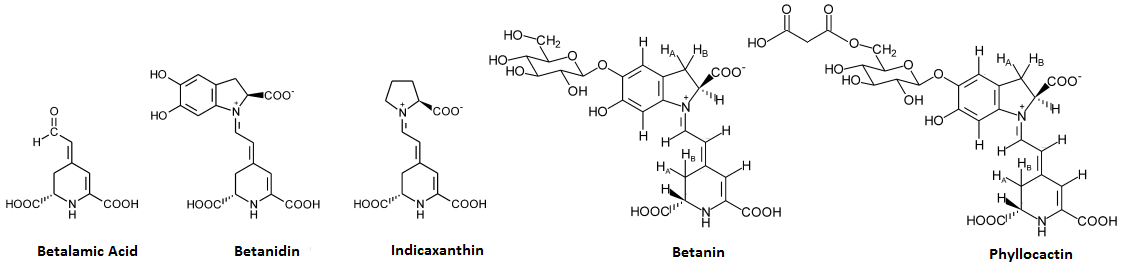
Most betalain structures are based off of the molecule known as betalamic acid, and two very similar structures to betalamic acid are betanidin and indicaxanthin; these three molecules form the majority of betalains as they are glycosylated (various sugars added) to form a variety of molecules
Similar to how proline is added to betalamic acid to form betanidin, the amino acid glycine can be added to betalamic acid to form portulacaxanthin III which can then have a Tyrosine added to it to form portulacaxanthin II;[1] a structually related betalain is dopaxanthin where the phenol group more closely resembles dopamine, or there is miraxanthin V which is structually dopamine-betalamic acid.[1] Tryptophan-betalamic acid (Tryptophan-betaxanthin) also exists alongisde a betxanthin for GABA, serine, phenylalanine, and each of the branched chain amino acids.
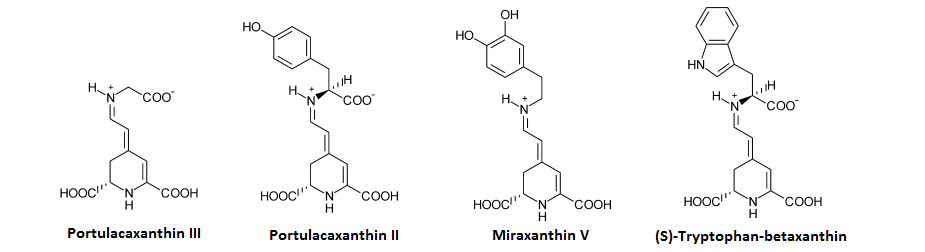
Rather than forming betanidin (which requires a proline to be attached), betalamic acid can also attach to a variety of other small amino acids which include most of the neurologically active amino acids
1.3
Physicochemical Properties
The betalains are water soluble and stable in a pH range of 3-7[1] and possess ionizable carboxyl groups and a positive charge on the nitrogen molecules (on the proline)[12] and due to this they can be classified as zwitterions like Trimethylglycine.[13][14]
2.
Pharmacology
2.1
Absorption
In isolated Caco-2 cells at a concentration of 100µM, betanin and indicaxanthin do not appear to be significantly metabolized and are both absorbed[12] with the absorptive phase being higher than efflux.[12]
While indicaxanthin does not appear to have its absorption altered when transporters (MCT1 and OATP2B1) are competitively inhibited, betanin has its absorption increased in the presence of valproate[12] which is a substrate for both[15] and by indomethacin which is an inhibited of MRP2.[12]
Opening tight junctions drastically increases the absorption of both, suggesting paracellular absorption also plays a role.[12]
Betalain absorption appears to have both transporter mediated uptake as well as a paracellular/passive (in between cells) component
Absorption of betalains (betanin and indicaxanthin) from a food product (cactus pear undergoing simulated digestion) indicaxanthin appears to have similar absorption profiles to free indicaxanthin also betanin was reduced somewhat.[12] The authors hypothesized the additional phenolic group and/or glucoside was interacting with other components of the food product to reduce absorption.[12]
Indicaxanthin appears to be as well absorbed from food products as it is in free form (in vitro evidence) whereas betanin may be hindered somewhat
2.2
Serum
Consumption of beet juice[13][14] or other sources of betalains such as cactus pear fruit[16] can increase urinary concentrations of these betalains (unmetabolized), suggesting a serum exposure.
Oral ingestion of 500g of fresh cactus pear fruit has resulted in peak plasma betalain (betanin and indicaxanthin, but not betanidin) concentrations after three hours and detectable within one hour.[16] The half life for betanin (0.94+/-0.07 hours) was significantly less than that of indicaxanthin (2.36+/-0.17 hours).[16]
2.3
Elimination
Betalains appear to be eliminated in the urine with a mean time of 4.2+/-2.3 hours after oral ingestion of juice (range of 2-8.5 hours) following consumption of juice[13] and has accounted for 0.21–0.39% of the oral dose[13] similar to other studies measuring 0.28% in urine following beet ingestion[14] yet up to 3.7% with cactus pear fruit pulp;[16] it is thought[12] that this reflects poor absorption, and cactus fruit is higher due to higher relative levels of indicaxanthin relative to betanin.
When investigating indicaxanthin alone, it appears that oral ingestion of 28mg indixacanthin and measuring the urine over 12 hours results in 76+/-3% of the total oral dose being detected; suggesting good absorption and rapid elimination.[16]
Although there appear to be urinary metabolites of betalains (which may color the urine red following ingestion of beets), it appears to be low relative to the overall oral ingestion suggesting poor bioavailability
2.4
Phase II Enzyme Interactions
Oral ingestion of 10-150ppm of beet pigments in the rodent diet (10-150mg/kg) for two months was able to increase antioxidative capacity in rats associated with the red and purplish pigments (but not orange-yellow nor brown colored betalains) was able to increase quinone reductase activity;[17] the fractions that appeared to be more active contains vulgaxanthins, and the dose was stated to be equivalent to one beet consumption daily.[17]
3.
Cardiovascular Health
3.1
Red Blood Cells
Indicaxanthin has been detected in red blood cells at a peak concentration of 1.03+/-0.2μM three hours after consumption of the fruits of cactus pear, which was halved in concentration after five hours;[18] betanin was found only to the level of 30.0+/-5.2 nM after three hours and was undetectable at five hours[18] and when tested ex vivo concentrations of total betalains at 5μM or higher reduce oxidative damage and hemolysis of red blood cells.[18]
3.2
Atherosclerosis
Betalains can bind to LDL[19] and the oxidation of LDL appears to be inhibited by both betanidin and betanin (IC50 of less than 2.5μM)[14] which is more potent than Vitamin E but may also act synergistically with Vitamin E.[19]
Following oral consumption of cactus pear fruit at 500g fresh weight (28mg indicaxanthin and 16mg betanin) LDL particles appeared to accumulate total betalains at 3 hours (100.5+/-11pM/mg protein) and 5 hours (50+/-7.2 pM/mg) resulting in more resistance of LDL to oxidation when tested ex vivo.[16]
Betalains appear to hinder LDL oxidation, and this has been noted 3-5 hours after oral ingestion of cactus pear fruit (high indicaxanthin content)
4.
Interactions with Oxidation
4.1
General
Betanin and Betanidin appear to be able to inhibit lipid peroxidation ex vivo (IC50 value of 400nM and 800nM; respectively) with a potency greater than both catechin (1.2μM) and Vitamin E (5μM)[14] and was also able to inhibit LDL oxidation with a IC50 value less than 2.5μM, again more potent than catechin as reference.[14]
This antioxidative property of betalains appear to bleach the molecule (removing its pigmentation)[14] and coincubating betalains alongside other antioxidants (ie. glutathione) can prevent this[20] which suggests a role for the chromophore (betalamic acid) itself in the antioxidant properties of betalain molecules.
Betanidin and Betanin appear to be quite potent antioxidants against lipid peroxidation (the oxidation of lipids and fatty acids) and may be active despite their low absorption rates in humans
4.2
DNA Damage
Betalain has been reported to react with peroxynitrate (ONOO-) in vitro,[20] and due to ONOO- being one of the more potent nitrosylative oxidants[21] and capable of damaging DNA[22] incubation with betalains (from beets; mostly betanin and isobetanin) reduced this damage with an IC50 of 19.2µM; comparable potency to blueberry anthocyanins (13.8µM) and greater than Vitamin C (79.6µM).[20]
This process may require to carboxyl group of the cyclo-dopa group (the part of betanin which is not betalamic acid) becoming nitrosylated and deattaching itself from betalamic acid.[20]
Appears to scavenge peroxynitrate radicals with a potency comparable to anthocyanins from blueberry
5.
Peripheral Organ Systems
6.
Safety and Toxicology
6.1
False Positives
Due to the red pigmentation of some betalains (betanidin and betanin) found in beetroot and the lack of metabolism in the body they may experience, these betalains are known to color both the urine and feces a bright red color. This can reduce in concerns related to both rhabdomyolysis (a kidney injury associated with blood in the urine) or colon damage (due to presence of what looks like a blood color in the feces).
To properly assess the color of the urine and feces, give at least one and three days (respectively) with no betalain consumption to clear the body of any significant betalain content.


