Does the sweetener erythritol increase the risk of cardiovascular disease? Original paper
A series of studies reported that high levels of the sweetener erythritol in the blood were associated with an increased risk of CVD events, and erythritol may have pro-coagulant effects. Whether these findings are sufficient to consider erythritol as a cause of CVD remains debatable.
This Study Summary was published on April 3, 2023.
Quick Summary
A series of studies reported that high levels of the sweetener erythritol in the blood were associated with an increased risk of CVD events, and erythritol may have pro-coagulant effects. Whether these findings are sufficient to consider erythritol as a cause of CVD remains debatable.
What was studied?
Whether blood levels of erythritol are associated with cardiovascular disease events and, if so, what mechanism might explain such an association.
Who was studied?
This study involved multiple separate cohorts:
- Discovery cohort: 1,157 participants (average age of 65) who were undergoing elective diagnostic cardiac evaluation. Of this group, 75.5% had coronary artery disease, 16.7% had heart failure, and 46.3% had experienced a heart attack.
- United States (U.S.) cohort: 2,149 participants (average age of 63). A total of 75% had coronary artery disease, 19.4% had heart failure, and 39.5% had experienced a heart attack.
- European cohort: 833 participants (average age of 75). A total of 69.3% had coronary artery disease, 17.8% had heart failure, and 49.6% had experienced a heart attack. -Experimental cohort: 8 healthy participants.
How was it studied?
The investigators performed a metabolomics analysis using the discovery cohort, assessing the association between 41 compounds (polyols and polyol metabolites) and the risk of major adverse cardiovascular events (MACE), defined as death, nonfatal heart attacks, and nonfatal strokes, over 3 years of follow-up.
Using both the U.S. cohort and the European cohort, the investigators assessed the association between the metabolite most strongly associated with MACE (erythritol) during 3 years of follow-up. The analyses were adjusted for various potential confounders, namely, age, sex, diabetes, systolic blood pressure, LDL-C, HDL-C, triglycerides, and smoking. Additionally, an adjustment for BMI was made in the U.S. cohort.
The investigators also performed experiments to determine whether erythritol would make blood more likely to clot.
In a series of in vitro experiments, the investigators incubated human platelet-rich plasma with erythritol to see whether it would enhance platelet aggregation (a marker of clotting tendency) in response to several promoters of platelet aggregation. This involved testing different levels of erythritol to see at what threshold any effects might occur.
In additional in vitro experiments, the investigators performed experiments on human blood to determine whether erythritol at a concentration of 45 μM would promote platelet adhesion (another marker of clotting tendency) in response to collagen (a promoter of platelet adhesion) over the course of 3 minutes. This experiment was performed 10 times with erythritol, 11 times with a control, and 3 times without collagen.
In animal experiments, the investigators injected 12 mice with erythritol (25 mg/kg), 11 mice with a control substance (1,5-AHG, 25 mg/kg), and 8 mice with saline. The mice then had their carotid artery injured, with investigators examining whether erythritol stopped the bleeding more quickly, with the time until cessation of blood flow used as a marker of clotting tendency.
Finally, using the experimental cohort, investigators assessed whether ingestion of 30 grams of erythritol would increase erythritol to the levels able to increase clotting tendency in the in vitro experiments. Erythritol levels were assessed 30 minutes, 2 hours, 6 hours, 1 day, 2 days, 3 days, 4 days, and 7 days after consumption.
What were the results?
Discovery cohort: In the discovery cohort, blood levels of erythritol were strongly associated with the risk of MACE, although other compounds associated with a higher risk of MACE included isothreonic acid, threitol, pseudouridine, arabitol, myo-inositol, xylose, cellobiose, mannose, levoglucosan, and saccharic acid (glucaric acid).
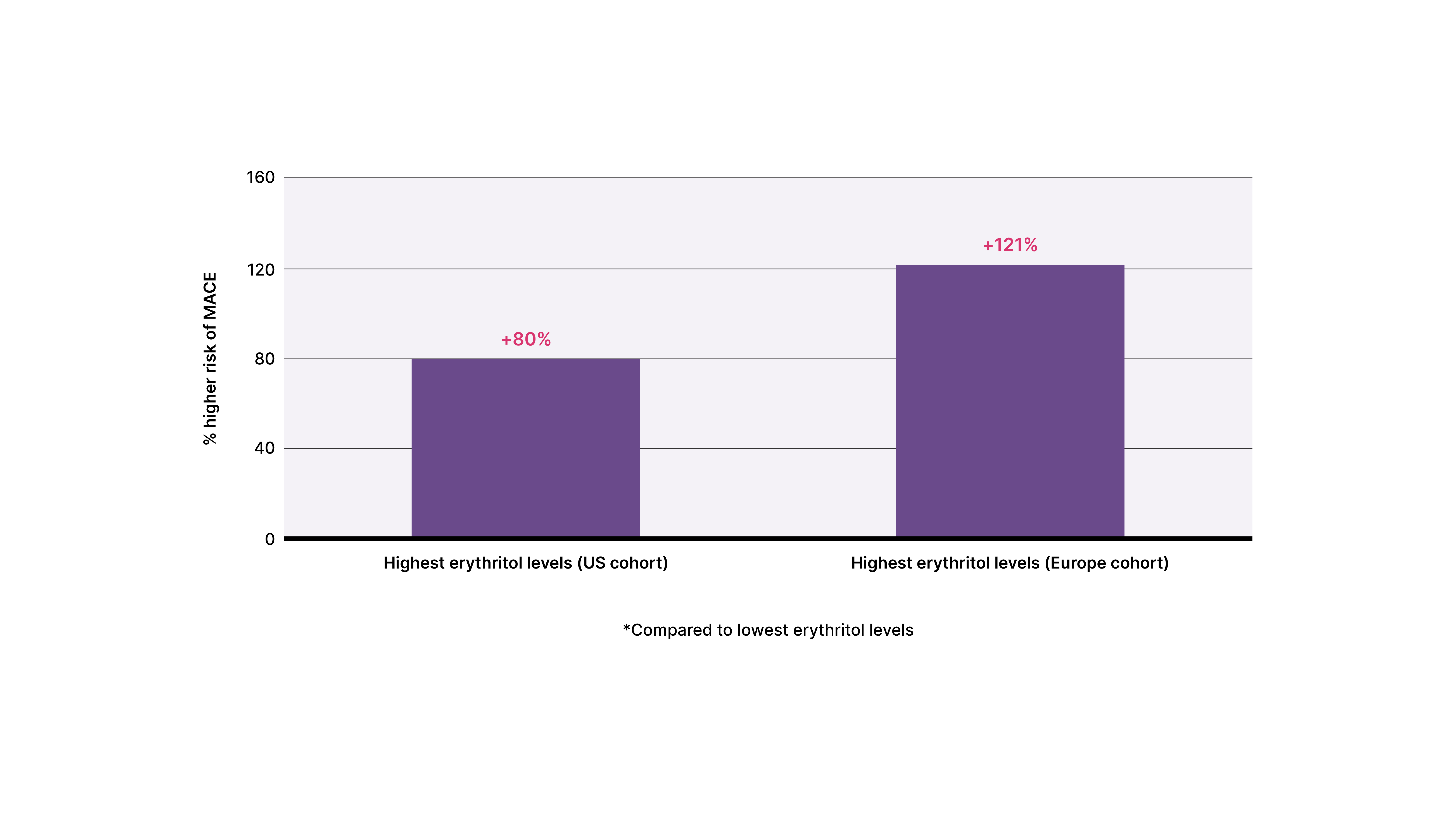
U.S. and European cohorts: In both the U.S. and European cohorts, participants in the top 25th percentile for blood erythritol had a higher risk of MACE compared to participants in the lowest 25th percentile — 80% higher in the U.S. cohort and 121% higher in the European cohort.
Serum erythritol levels and risk of Major Adverse Cardiovascular Events (MACE)
In vitro and mouse studies: Incubation of platelet-rich plasma with erythritol promoted platelet aggregation and, similarly, incubation of whole blood with erythritol promoted platelet adhesion. Mice injected with erythritol had their bleeding stop more quickly compared to the control or saline injection, indicating a greater tendency towards clotting.
Experimental cohort: Consuming 30 grams of erythritol increased blood erythritol levels above three thresholds (45 μM, 18 μM, and 4.5 μM) shown in vitro to promote different aspects of platelet responsiveness (adhesion or aggregation). Notably, all participants had erythritol levels above the highest threshold for one day afterward, most participants had levels above the intermediate threshold for two days afterwards, and most participants had levels above the lowest threshold for three days afterwards.
The big picture
Using three separate cohorts, this study found an association between high erythritol levels and CVD events, but do these results suggest that eating erythritol increases the risk of CVD? Not necessarily — the investigators only looked at erythritol levels in the blood, meaning that it’s not known how much erythritol the participants were actually ingesting. This is important because erythritol levels can be influenced by other factors beyond just ingestion.
One determinant of blood erythritol levels is endogenous synthesis. Erythritol is made in the body, synthesized via the pentose phosphate pathway (PPP).[1] A major purpose of the PPP is to generate a molecule called NADPH as a way of protecting against oxidative stress. NADPH activates various antioxidant pathways in the body.[2] In other words, rather than erythritol causing CVD, higher erythritol levels in the blood could very well be an indicator of poor health and higher oxidative stress.
Another important determinant of blood erythritol levels is urinary excretion.[3] The major route by which erythritol is removed from the body is via the urine, meaning that reduced kidney function could result in higher levels of erythritol in the blood. Research in this area is limited, but at least one study on children with chronic kidney disease suggested that lesser kidney function does indeed reduce erythritol excretion.[4] So higher erythritol levels could be a marker of reduced kidney function, which is notable because reduced kidney function is associated with a high risk of CVD.[5][6]
Therefore, there are compelling theoretical explanations for why erythritol in the blood would be associated with CVD but not actually promote CVD. But what about the evidence suggesting that erythritol promotes clotting?
Altering clotting tendency is a feasible mechanism by which a substance could affect CVD risk. Anticoagulant drugs like warfarin and antiplatelet drugs like aspirin are often given to people at high risk of CVD for this very reason. They can prevent blood clots from forming and causing a heart attack or stroke. On the other hand, mechanistic evidence on how a substance affects clotting tendency does not always translate into real clinical effects. An example of this is vitamin E supplementation. Vitamin E seems to inhibit clotting tendency based on in vitro studies,[7] but when actually tested in multiple large randomized controlled trials, high doses of vitamin E have not been found to decrease the risk of cardiovascular disease.[8] Even aspirin does not always reduce the risk of CVD, with its benefits appearing questionable if risk is not sufficiently high.[9]
Another example of the limitations of the evidence implicating erythritol in causing CVD has to do with a substance called trimethylamine N-oxide (TMAO). TMAO is a chemical produced in the human body, with greater amounts usually made when a person eats foods high in choline, like eggs, and carnitine, like red meat. In 2016, a research group, including several researchers from the current erythritol study, published a study in Cell showing that, similar to in the erythritol paper, higher blood levels of TMAO are linked to a higher risk of CVD events and that TMAO increases platelet aggregation in vitro and in mice.[10] However, in the years since this study was conducted, several inconsistencies have cast serious doubt on the idea that TMAO promotes heart disease, including the following:
- Eating fish seems to increase TMAO more than just about any other food,[11] yet eating fish also seems to decrease the risk of heart disease.[12]
- By and large, people with gene variants that increase their TMAO levels do not have a higher risk of heart disease.[13]
Currently, many, although not all, researchers now suspect TMAO is not itself harmful, but is instead more likely to be a benign marker of poor health. Both type 2 diabetes and kidney disease seem to result in higher TMAO levels, for example.[13] In other words, evidence implicating erythritol in promoting heart disease also once implicated TMAO, but subsequent research has called the TMAO-heart disease link into question.
Ultimately, this study doesn’t provide especially compelling evidence that consuming erythritol will increase the risk of CVD. That said, there are no long-term studies looking at the relationship between eating erythritol and CVD outcomes, making it difficult to completely rule out a possible harm of erythritol, at least among people at high risk of CVD, like the populations studied in this paper.
This Study Summary was published on April 3, 2023.
References
- ^Katie C Hootman, Jean-Pierre Trezzi, Lisa Kraemer, Lindsay S Burwell, Xiangyi Dong, Kristin A Guertin, Christian Jaeger, Patrick J Stover, Karsten Hiller, Patricia A CassanoErythritol is a pentose-phosphate pathway metabolite and associated with adiposity gain in young adultsProc Natl Acad Sci U S A.(2017 May 23)
- ^Stincone A, Prigione A, Cramer T, Wamelink MM, Campbell K, Cheung E, Olin-Sandoval V, Grüning NM, Krüger A, Tauqeer Alam M, Keller MA, Breitenbach M, Brindle KM, Rabinowitz JD, Ralser MThe return of metabolism: biochemistry and physiology of the pentose phosphate pathway.Biol Rev Camb Philos Soc.(2015-Aug)
- ^Mazi TA, Stanhope KLErythritol: An In-Depth Discussion of Its Potential to Be a Beneficial Dietary Component.Nutrients.(2023-Jan-01)
- ^Vanlede K, Kluijtmans LA, Monnens L, Levtchenko EUrinary excretion of polyols and sugars in children with chronic kidney disease.Pediatr Nephrol.(2015-Sep)
- ^Wang Y, Katzmarzyk PT, Horswell R, Zhao W, Johnson J, Hu GKidney function and the risk of cardiovascular disease in patients with type 2 diabetes.Kidney Int.(2014-May)
- ^Said S, Hernandez GTThe link between chronic kidney disease and cardiovascular disease.J Nephropathol.(2014-Jul)
- ^Steiner MVitamin E, a modifier of platelet function: rationale and use in cardiovascular and cerebrovascular disease.Nutr Rev.(1999-Oct)
- ^Eidelman RS, Hollar D, Hebert PR, Lamas GA, Hennekens CHRandomized trials of vitamin E in the treatment and prevention of cardiovascular disease.Arch Intern Med.(2004-Jul-26)
- ^Berger JSAspirin for Primary Prevention-Time to Rethink Our Approach.JAMA Netw Open.(2022-Apr-01)
- ^Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SLGut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk.Cell.(2016-Mar-24)
- ^Lombardo M, Aulisa G, Marcon D, Rizzo G, Tarsisano MG, Di Renzo L, Federici M, Caprio M, De Lorenzo AAssociation of Urinary and Plasma Levels of Trimethylamine N-Oxide (TMAO) with Foods.Nutrients.(2021-Apr-23)
- ^Bo Zhang, Ke Xiong, Jing Cai, Aiguo MaFish Consumption and Coronary Heart Disease: A Meta-AnalysisNutrients.(2020 Jul 29)
- ^Jia J, Dou P, Gao M, Kong X, Li C, Liu Z, Huang TAssessment of Causal Direction Between Gut Microbiota-Dependent Metabolites and Cardiometabolic Health: A Bidirectional Mendelian Randomization AnalysisDiabetes.(2019 Sep)
